OVerview
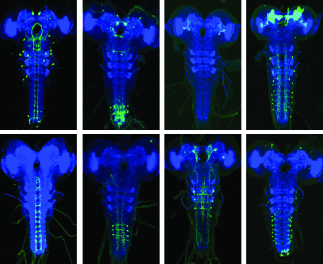
Our lab's primary research goal is to understand the circuit principles by which behavioral choices are made in the nervous system. We are interested in how animals select specific modes of locomotion and sequences, based on information from multiple modalities, prior experience, and internal states. We use Drosophila larvae to address this question at three levels of analysis: behavior, physiology, and connectome. The exquisite genetic toolkit in Drosophila is available such as selective manipulation of a large fraction of individual neuron types in the larval nervous system. We have developed automated behavior assays to test the effects of such manipulation on behavioral choices. Furthermore, the small size of the nervous system (only 10,000 neurons) makes it possible to reconstruct the neuronal connectivity from electron microscopy stacks—an ongoing collaborative project in the Drosophila larval community spearheaded by my key collaborator at JRC, Dr. Albert Cardona. Finally, physiology/imaging techniques with optogenetic enable functional connectivity mapping with anatomically defined circuits. Using cutting edge of techniques in neuroscience, our lab will tackle how the nervous system (1) integrates information to determine the final behavioral output, (2) generates properly ordered action sequences, and (3) changes when genetic modifications alter behavior.
1. Elucidation of integration mechanisms at command-like neurons:
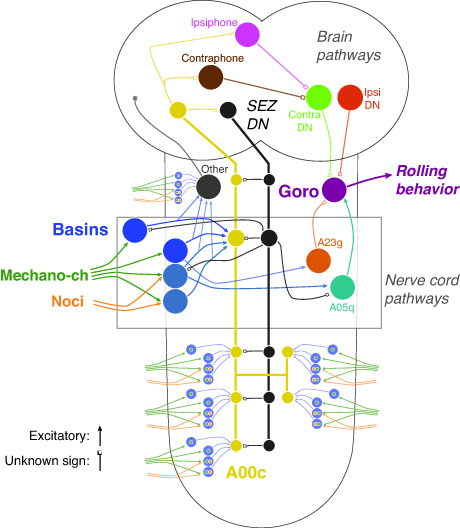
The function of a neural network cannot be determined solely by knowing how its components are wired together. Knowing their active properties is also crucial. Although my recent work has shown where mechanical and nociceptive inputs converge in the larval nervous system and that their convergence at multiple levels could in principle explain their tunable inputs and output combination, how the properties of neurons contribute to action selection remain to be tested. I will focus on the command-like Goro neuron, which is positioned to integrate local and global multimodal information from the ventral nerve cord and brain, respectively. EM reconstruction of the neurons that provide inputs onto Goro neurons reveal that a single Goro neuron receives more than 500 input synapses from more than 30 different cell types. I will examine the integration mechanisms at Goro neurons by using imaging physiology and electrophysiology in combination with optogenetic activation of circuit elements. I will also investigate the molecular mechanisms that underlie the integration of multimodal inputs at the command-like neurons by using RNAi knock down.
2. Analysis of action sequences:
Many complex animal behaviors that are crucial for survival consist of sequences of properly ordered actions, as do many skills learned by humans. Serial and parallel top-down models have been proposed to explain the generation of action sequences. In the former class of model (e.g., a synfire chain), the activation of an action module (i.e., a circuit devoted to producing a specific action) activates the next module. Some support for this hypothesis comes from studies on the mechanisms of birdsong. An alternative class of model assumes hierarchical suppression (mediated by asymmetric mutual inhibition) among action modules activated in parallel. However, whether and how neural circuits actually implement such mechanisms remains unclear. I will address these questions by uncovering the circuit mechanisms underlying the Drosophila larval action sequence of “rolling followed shortly by fast crawling.” I have found that activating a subset of Basin cells—the first interneurons that integrate inputs from nociceptive and mechanosensory neurons—is necessary and sufficient for rolling and subsequent fast crawling, whereas activating the command-like Goro neurons induces only rolling. This finding is consistent with hierarchical suppression. Using candidate neurons that I have identified to control fast crawling, I will identify the neurons that inhibit fast crawling elements and which are controlled by the rolling circuit.
3. Evolution of neural circuits and behavior:
The relationship between the evolution of neural circuitry and that of behavior is not well understood. The properties of neural circuits are thought to affect the evolvability of behavior. For example, it has been suggested that the evolution of particular behaviors may be constrained or promoted by the organization of neural circuits. The components of the neural circuits that we have identified for escape behaviors in Drosophila melanogaster offer a potential opportunity to dissect the relationship between the evolution of behavior and that of neural circuits. In preliminary experiments, I have tested the responses of several Drosophila species to noxious stimuli and found that the probability of rolling varies widely. To determine the sources of this variation, I plan to generate EM stacks and reconstruct the wiring diagrams in selected Drosophila species. I also plan to compare the molecular, physiological, and behavioral phenotypes in first-order interneurons and a command-like neuron across in these species by using electrophysiology, RNA seq, and high-throughput behavior analysis in combination with tools provided by D. Stern (JRC, unpublished). These experiments should begin to shed light on what changes in molecules and neural circuits may have driven behavioral changes during evolution.
In summary, my new lab will tackle how the nervous system (1) integrates information to determine the final behavioral output, (2) generates properly ordered action sequences, and (3) changes when genetic modifications alter behavior.